Context Matters
Bing Chen
Boston Children's Hospital
Published January 30, 2024
Bing Chen has spent his structural biology career seeking a better look at how HIV particles enter host cells to make people sick. In fact, his research group at Boston Children’s Hospital had leveled up to a new era of study with the full-length HIV fusion protein—not just the most active part that most studies have used for decades.
Then the COVID-19 pandemic was declared. A state-mandated lockdown of schools and non-essential services at universities forced his research group to choose: Shut down the lab and go home, or switch their focus to the pandemic virus, SARS-CoV-2.
The Chen lab members decided to pivot. They joined an international legion of like-minded scientists who turned their various expertise toward the new and urgent public health crisis. An HIV background turned out to be especially helpful.
With unexpected speed, Chen’s team applied their HIV techniques to express the full-length coronavirus spike fusion protein. They quickly solved their first coronavirus structure and deposited the details in the shared Protein Data Bank. Within three months, they submitted their first paper.
“It was quite surprising, remarkable, because of how long we have been working on HIV,” Chen says. “It took us more than 10 years even to produce a well-behaved full-length HIV envelope protein.”
After their first coronavirus structure, the group chased down the spike structures of newly arising variants – Alpha, Beta, Gamma, Kappa, Delta and Omicron (BA.1 and BA.2). They found the structural basis for enhanced infectivity and immune evasion as the virus evolved. By the official end of the pandemic, they had published 4 papers in the journal Science on the structures and their implications.
”Once we got to Omicron, we decided that's enough,” Chen says. “That's not going to be useful, just chasing after all these different new variants.”
Instead, Chen noticed, the many structures of the spike protein solved by his group and others, were all missing the pieces that interacted with the membrane. Those pieces may have been deleted intentionally because of the difficulty in working with them, or as in Chen’s case, they were disordered because the proteins were dissolved in aqueous solutions containing detergent.
As with HIV, he was convinced that the full fusion story could only be told in the context of the membranes the spike protein was joining together.
“You can imagine the main function of these proteins is to catalyze the membrane fusion, and yet we don't have any structure of these membrane-interacting segments,” he said. “It's almost like you're studying an enzyme, and you don't have the active site.”
So they reconstituted the membrane protein packed in an experimental lipid bilayer called a nanodisc, which they had been doing with the HIV fusion protein before the pandemic interrupted. In the case of SARS-CoV-2, “if you mix this preparation with the receptor, ACE2, that can trigger the protein to convert to the postfusion structure,” Chen says.
The structure of postfusion conformation revealed the real fusion peptide. Called FP for short, the segment inserts into the target cell membrane once the spike is activated (Nature, 2023).
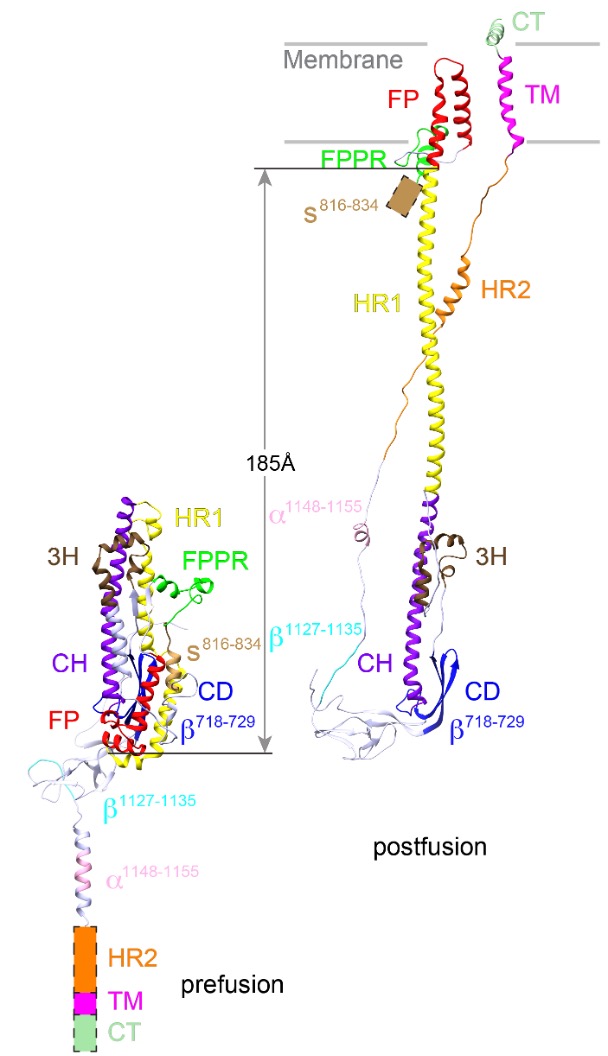
In this single strand of the trimer spike fusion protein of SARS-CoV-2, the internal fusion peptide (FP in red) forms a hairpin-like wedge that spans almost the entire lipid bilayer, and the transmembrane segment (TM in pink) wraps around the fusion peptide at the last stage of membrane fusion.
“Our results show unambiguously that only the FP interacts with membrane,” wrote postdoctoral fellow Wei Shi and his co-authors. “Our results underscore the risk of studying fusion peptides when taken out of the context of intact viral fusion proteins, because many hydrophobic peptides can induce structural changes of a lipid bilayer.”
After the worst of the pandemic had passed, the Chen lab resumed its work on HIV, buoyed by the successes in their coronavirus structures. Moving forward, the lab will work on both viruses. No vaccine exists yet for HIV, and the world will need useful tools if another coronavirus emerges to sicken people, Chen says.
Beyond the basic science of understanding the complicated fusion machinery, Chen is interested in translational aspects, such as developing vaccines and small-molecule antiviral therapeutics to block the membrane fusion process.
The different fusion proteins used by HIV and SARS CoV-2 are more similar than one might think. “They have totally different sequences and recognize different receptors and all that,” Chen says, “but they have pretty similar overall organization of the protein complex.”
And both proteins practice the same calisthenic routine to fuse with healthy cells. The proteins unfold and stretch out to grab the cell membrane. Then, the protein doubles over, pulling the virion and cell membranes together to create an opening and spill in the viral contents.
The dramatic changes in shape have big implications for vaccines and therapeutics.
In HIV, for example, although people infected with HIV produce many antibodies against the protein encapsulating the virus, most of these antibodies are strangely ineffective at fighting the disease. Chen’s group found that a rare group of useful neutralizing antibodies home in on the fleeting fusion-intermediate shape, just before the virus enters a cell. (Proc Natl Acad Sci 2008). The other antibodies mount a futile defense against the postfusion shape, when it’s too late (Nature Structural & Molecular Biology, 2010).
(The COVID-19 mRNA vaccines work by teaching cells how to make a protein that triggers an immune response against the spike protein in its prefusion state.)
Chen grew up in China in a remote city close to Mongolia. In high school, he read a biochemistry textbook he found in the local bookstore. He became fascinated with how biochemists could explain the mysteries of life. (Since becoming a scientist, he has discovered that each mystery solved leads to new mysteries. “The more we learn, the more we know we don’t know,” he says.)
At the time in China, an aspiring student had to choose a major when applying to college after passing an entrance exam. In a tailor-made opportunity, Fudan University in Shanghai was recruiting students to major in biochemistry. It was a time when biology resources were scarce, Chen recalls.
Chen headed to Ohio State University in Columbus for a PhD and studied RNA biochemistry. When it came time to consider a postdoctoral position, he had the option to continue working on RNA or do something different. But he had witnessed the tedious process of mutating RNA residue by residue to try to figure out how it interacted with a certain protein.
Solving a crystal structure was much more appealing. He joined the shared labs of Don Wiley and Stephen Harrison, then on the Harvard University campus in Cambridge. Before Chen arrived, researchers in Wiley’s lab had discovered the dramatic shape changes influenza’s haemagglutinin protein underwent for viral entry into cells. But it was decades between solving the structures of the pre- and post-fusion shapes.
In contrast, in Chen’s lab during the pandemic, when postdoctoral fellow Yongfei Cai first purified the spike protein, he thought he had failed because of the multiple peaks on the chromatography readout. But he soon realized that he was looking at both prefusion and postfusion conformations.
“That was sort of really a dream come true for people working on the virus entry problem,” Chen says, “because often you spend years and years working on one conformation to capture another confirmation, and then we had the two in the same preparation.”
Chen started an independent lab in 2006 at Boston Children’s Hospital. Then, he primarily used x-ray crystallography for structure determination. Now, he works exclusively with cryo-electron microscopy, thanks to recent technological advances that allow them to tackle challenging problems, such as the full-length viral fusion proteins in membrane. He collaborates with several colleagues in the Harvard medical community, such as Duane Wesemann, Michael Seaman, Dan Barouch and Frederick Alt on HIV vaccine development.
“I think the lipid bilayer, the membrane, is going to be quite important in terms of stabilizing the specific conformations of the protein, which is important for thinking about the vaccine immunogen design,” Chen says. “You want to have the right conformation to present the immune system in order to induce relevant antibody responses.”
-Carol Cruzan Morton